
However, they are now known as 1, 2, 3,…18. Previously, the groups were referred to as IA,…VIIIA, VIII, IB…VIIB, and 0. Groups are formed by elements that have atoms with similar outer shell electrical structures. In this type of periodic table, the horizontal rows are known as periods, while the vertical columns are known as groups. The current version of the periodic table is the long version, which is commonly used all around the world. Hence their characteristics are different.Īlso read: Periodic Table of Elements – Trends and Patterns Modern periodic table of the elements – Long Form All elements have distinct electronic configurations over time.E.g., Three shells are present in a third-period element (K, L, M). The number of shells in an element’s atoms determines its period number.Inert gases, often known as Noble gases, are elements with fully occupied valence shells.
#Periodic table groups and periods series#
It’s also listed individually with the lanthanide series at the bottom of the main table. Actinides are elements with atomic numbers ranging from 90 to 103, and the series is known as the actinide series.Lanthanides are elements with atomic numbers ranging from 58 to 71, and the series is known as the Lanthanide series.It consists of elements from groups 3 through 12. The outermost shell (next to the outermost shell-penultimate shell) and the second outermost shell (next to the outer shell-penultimate shell) are missing.It consists of groups 1 and 2 (alkali metals) and 13 and 17 (alkaline earth metals).Only the outermost shell is empty the interior shells are all filled. Because each group’s elements have the same amount of valence electrons, their properties are identical. 1,2,3,…18 are the names or numbers of the 18 vertical columns.It is made up of 18 vertical columns and 7 horizontal rows. In its current form, the periodic table is known as the modern periodic table. The table is an arrangement of elements in increasing atomic number order. The modern periodic table, also known as the long-form, is based on modern periodic law. This clarification increased the value of the law, which is still as widely used today as it was at the turn of the twentieth century when it expressed the only known relationship between the elements. In the years since great strides have been made in explaining the periodic law regarding the electronic structure of atoms and molecules.
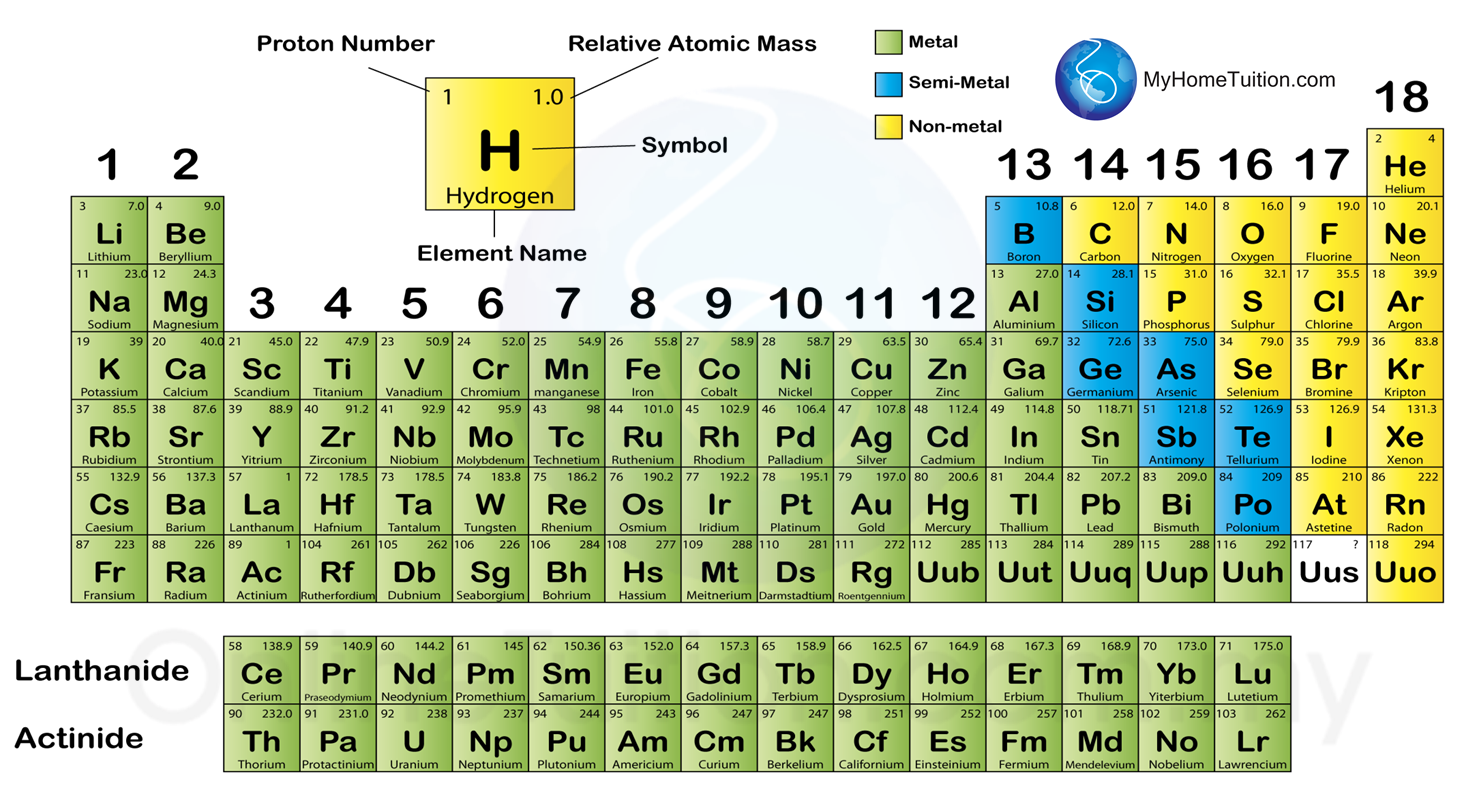
It wasn’t until the second decade of the twentieth century that it was realized that the periodic system’s order is defined by its atomic numbers, the integers of which are equal to the positive electrical charges of the atomic nuclei expressed in electronic units. Some scientific debate also rages about whether some elements in today’s table are correctly positioned. It is unknown how far the table will extend beyond these seven rows or whether the patterns of the known region will continue into this unknown region.

However, the chemical characterization of the heaviest elements is still required to confirm their properties match their positions. Today, all 118 elements are known, completing the first seven rows of the table. Only elements up to atomic number 94 exist in nature to go further, new elements had to be synthesized in the laboratory. With the advancement of science, the periodic table continues to evolve. The underlying cause of these trends is atomic electron configurations. In contrast, metallic character (surrendering electrons to other atoms) increases in the opposite direction.


 0 kommentar(er)
0 kommentar(er)
